Fabrication de produits de diagnostic in vitro
4.5 (427) · € 23.99 · En Stock
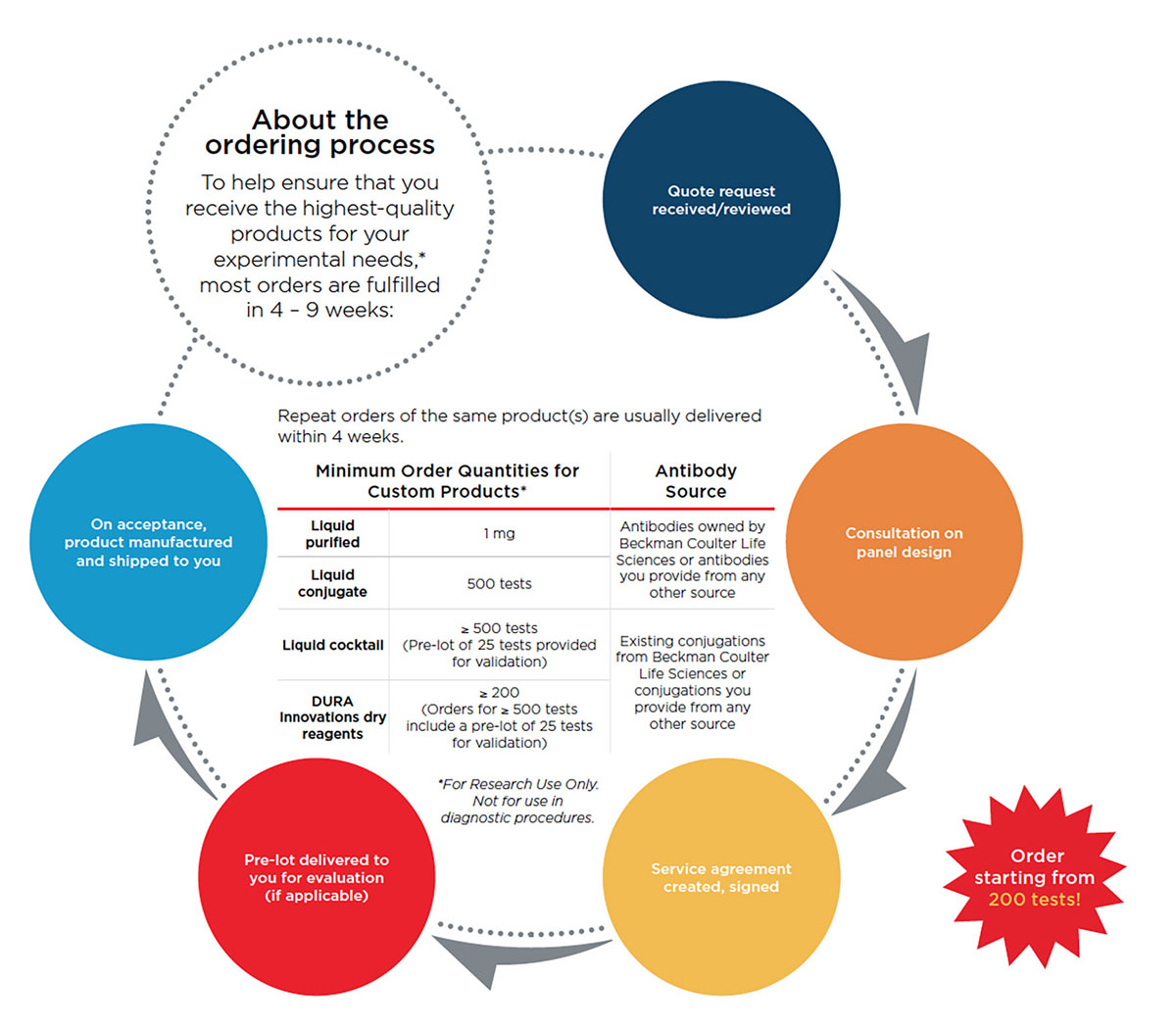
Contract Manufacturing Services - Bulk Orders Made-To
In Vitro Diagnostic Instruments

Réactifs pour construire votre propre analyse immunologique par

QuantStudio 5 Dx Real-Time PCR System (CE-IVD, IVDR)

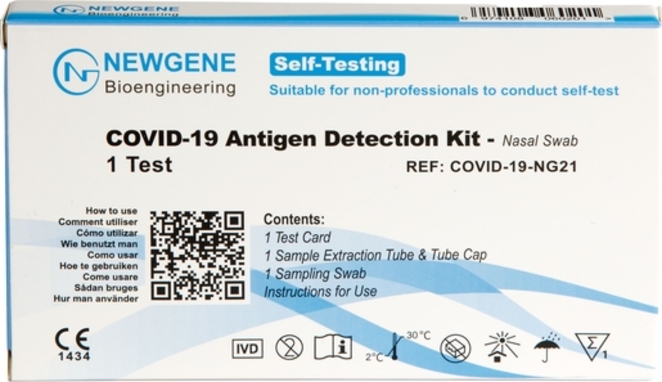
COVID-19 Testing Solutions Thermo Fisher Scientific - FR

SOBIODA, fournisseur de matériel de laboratoire de diagnostic

Companion Diagnostic (CDx) device under the IVDR update
ISO 18113-1:2022 - This document defines concepts, establishes general principles, and specifies essential requirements for information supplied by

ISO 18113-1:2022 - In vitro diagnostic medical devices — Information supplied by the manufacturer (labelling) — Part 1: Terms, definitions, and genera

Nova Biomedical

BIOTECanada Insights Spring 2023 by BIOTECanada - Issuu