Xpert® Xpress SARS-CoV-2 - FDA Emergency Use Authorization
4.9 (215) · € 14.00 · En Stock
Xpert® Xpress SARS-CoV-2/Flu/RSV received Emergency Use Authorization from the US FDA to support the global fight against COVID-19, with rapid detection of the current coronavirus SARS-CoV-2.

US FDA grants EUA for Cepheid's SARS-CoV-2 test

Cepheid on LinkedIn: Cepheid Receives Emergency Use Authorization from FDA for Rapid SARS-CoV-2…

Xpert Xpress XPRSARS-COV2-10 Sars-CoV-2 Reagent Cartridge - Henry Schein Medical

FDA grants 'emergency use' coronavirus test that can deliver results in 45 minutes

QIAGEN - Diagnostic companies are stepping up together to

FDA authorized molecular point-of-care SARS-CoV-2 tests: A critical review on principles, systems and clinical performances - ScienceDirect

Cepheid on LinkedIn: Cepheid Receives Emergency Use Authorization from FDA for Rapid SARS-CoV-2…

Cepheid Receives Emergency Use Authorization from FDA for Rapid SARS-CoV-2 Test - COVID-19 - mobile.

New COVID-19 Rapid Diagnostic Approved On 'GeneXpert' TB Platform; Could Pave Way For More Testing In Low- & Middle-Income Countries - Health Policy Watch

covid-19

Integrating tuberculosis and COVID-19 molecular testing in Lima, Peru: a cross-sectional, diagnostic accuracy study - The Lancet Microbe

Cepheid Developing Combination Test for SARS-CoV-2, Flu A/B, and RSV
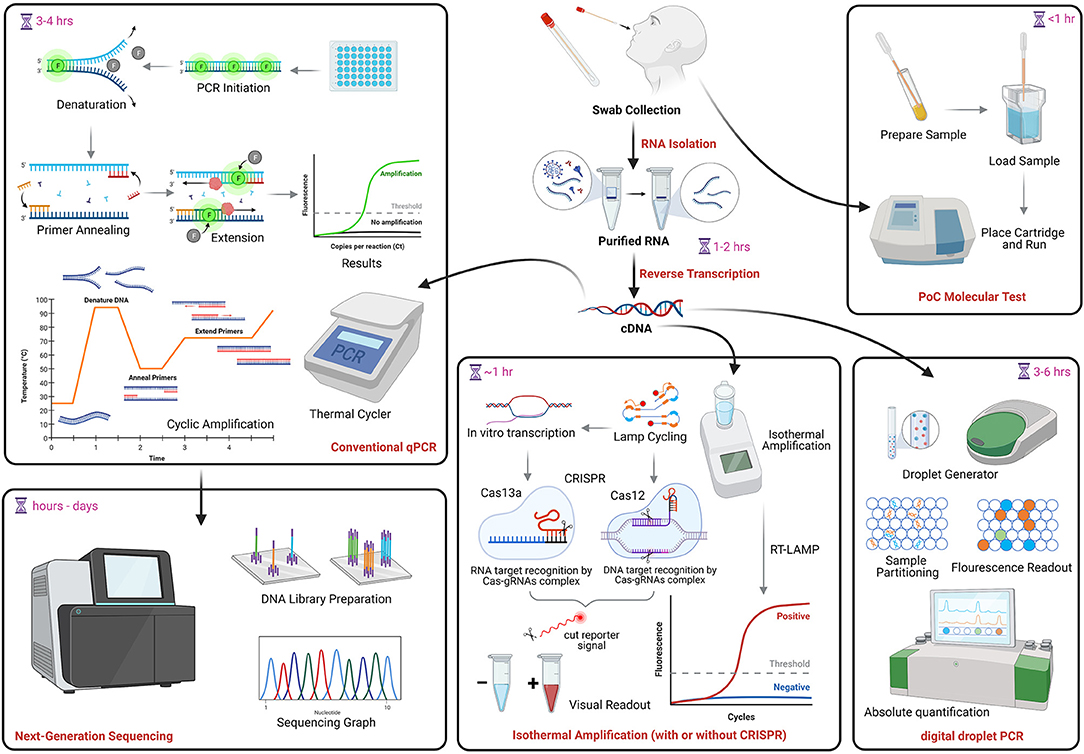
Frontiers Review of Current COVID-19 Diagnostics and Opportunities for Further Development










.png)