- Accueil
- g heat
- Ex.18 In the system, LaCl3(s) + H2O(g) + Heat = LaCIO(s) + 2HCl(g), is established. More water vapour is added to reestablish the equilibrium. The pressure of water vapour is doubled. The
Ex.18 In the system, LaCl3(s) + H2O(g) + Heat = LaCIO(s) + 2HCl(g), is established. More water vapour is added to reestablish the equilibrium. The pressure of water vapour is doubled. The
4.7 (640) · € 30.00 · En Stock
Click here:point_up_2:to get an answer to your question :writing_hand:ex18in the systemlacl3s h2og heat lacios 2hclgis established more water vapour is
Click here👆to get an answer to your question ✍️ Ex-18 In the system- LaCl3-s- - H2O-g- - Heat - LaCIO-s- - 2HCl-g- is established- More water vapour is added to reestablish the equilibrium- The pressure of water vapour is doubled- The factor by which pressure of HCl is changed is - -A-2 -B- V -C- 13 -D
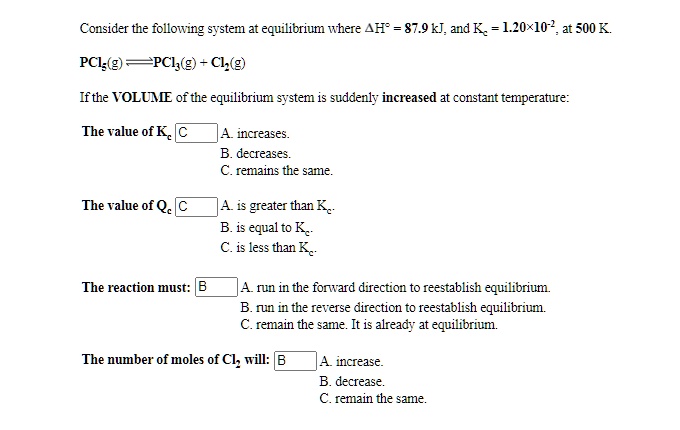
SOLVED: Consider the following system at equilibrium where ΔH° = 87.9 kJ, and K = 1.20*10^3, at 500 K. PCl(g) ⇌ PCl3(g) + Cl2(g) If the volume of the equilibrium system is
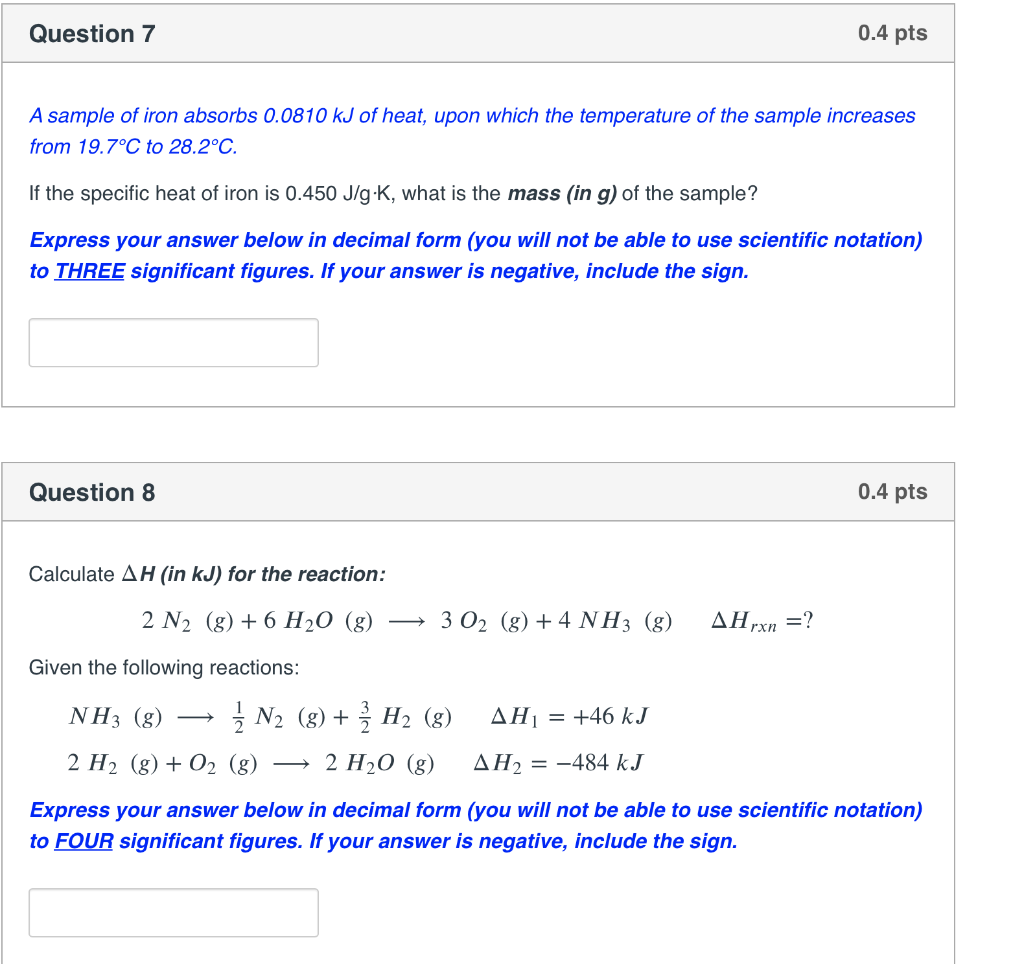
Solved Question 3 0.4 pts How much work (in J) does a gas

Thermochemistry and Thermodynamics - ppt download

Chemistry 2 Flashcards

Solved The vapor pressure P of a liquid rises exponentially

BT Hoa Ly 1-P2-Sv, PDF, Melting Point

Lecture 15. Phases of Pure Substances (Ch.5) Up to now we have dealt almost exclusively with systems consisting of a single phase. In this lecture, we. - ppt download

1) Consider the following reactions and their associated equilibrium - ppt download
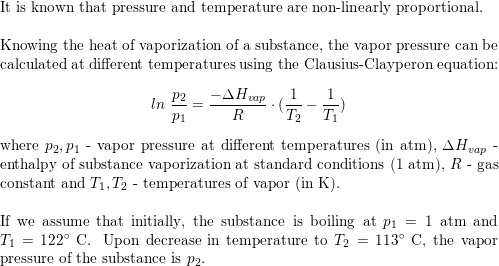

18.0 g of water completely vapourises at 100oC and 1 bar pressure and the enthalpy change in the process is 40.79 kj mol 1. What will be the enthalpy change for vapourising

Solved When the pressure is increased on the following

In the system, LaCl (s) + H2O(g) + heat= LaClO(s) + 2HCl(g). More water vapour is added to reestablish the equilibrium. The pressure of water vapour is doubled. The factor by









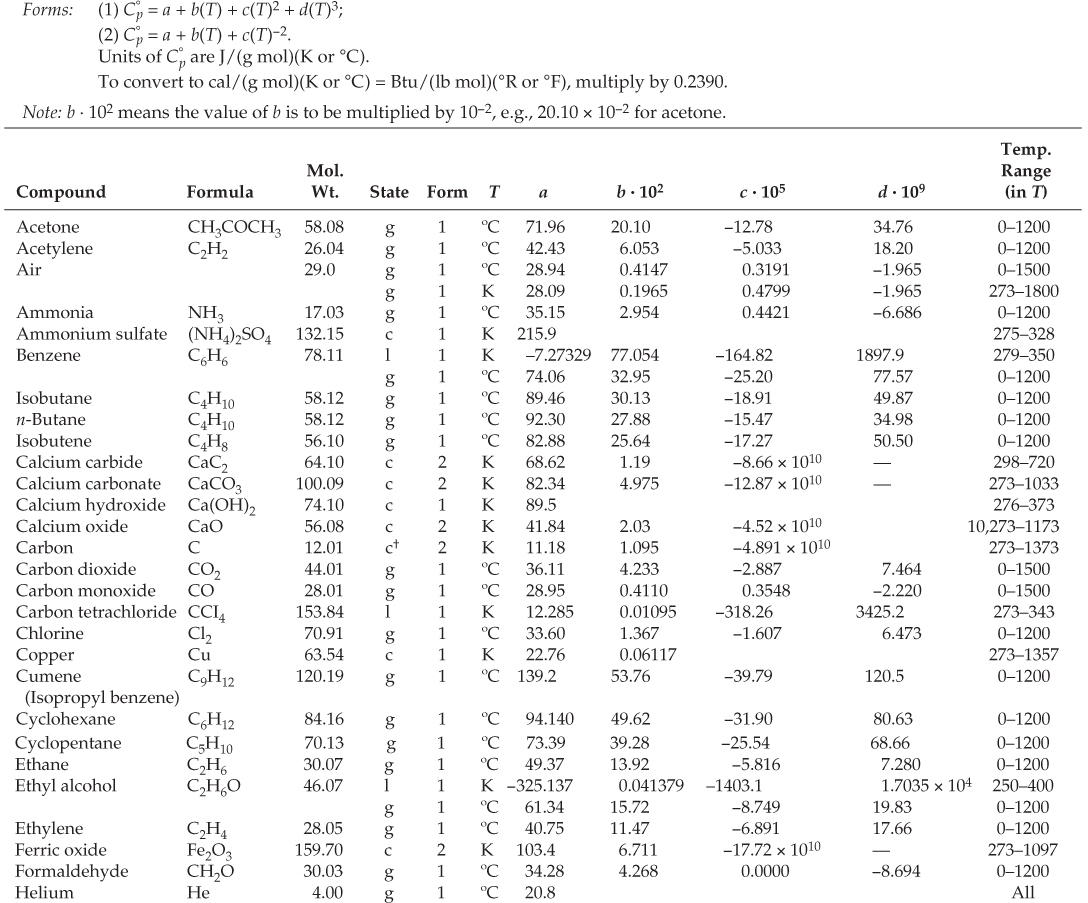

)
